Multiple Choice
|
|
|
1.
|
Which of the following compounds is
INSOLUBLE?
a) | lithium carbonate | b) | aluminum sulfide | c) | potassium
acetate | d) | magnesium bromide |
|
|
|
2.
|
Consider the following reaction: 2A
+ B  3C + D If 3.0 mol
A and 2.0 mol B react to form 4.0 mol C, what is the percent yield of this reaction?
|
|
|
3.
|
Which of the following would NOT be a product from
mixing hydrochloric acid with a solution of sodium sulfite?
a) | H2O (l) | b) | H2 (g) | c) | NaCl
(aq) | d) | SO2 (g) |
|
|
|
4.
|
Consider the reaction: 2 Al + 3 Br2
 2
AlBr3 2
AlBr3
Suppose a reaction vessel initially contains 5.0 mole Al and 6.0 mole
Br2. What is in the reaction vessel once the reaction has occurred to the fullest extent
possible?
a) | 2.0 mol Al; 3.0 mol Br2; 2.0 mol AlBr3 | b) | 0 mol Al; 1.0 mol
Br2; 4.0 mol AlBr3 | c) | 1.0 mol Al; 0 mol Br2; 4.0 mol
AlBr3 | d) | 0 mol Al; 0 mol Br2; 11.0 mol AlBr3 |
|
|
|
5.
|
If the actual yield of a reaction is 42.0 grams of
product and the percent yield is 75%, what is the theoretical yield?
a) | 56 g | b) | 0.56 g | c) | 1.8
g | d) | 32 g |
|
|
|
6.
|
Iron metal reacts with oxygen to produce iron(III)
oxide. If you have 12.0 moles of iron for complete reaction, you need:
a) | 12.0 mol of O2 and produce 24 mol of
Fe2O3. | b) | 4.5 mol of O2 and produce 3.0 mol of
Fe2O3. | c) | 9.0 mol of O2 and produce 6.0 mol of
Fe2O3. | d) | 9.0 mol of O2 and produce 3.0 mol of
Fe2O3. |
|
|
|
7.
|
How many moles of NH 3 are needed to react completely with 13.6 mol of
F 2, as shown below? a) | 5.44 mol | b) | 6.80 mol | c) | 34.0
mol | d) | 2.27 mol |
|
|
|
8.
|
A precipitate is expected to be formed when an
aqueous solution of sodium sulfate is added to an aqueous solution of:
a) | iron(III) chloride | b) | potassium chloride | c) | magnesium
chloride | d) | barium chloride |
|
|
|
9.
|
How many grams of oxygen are needed to completely react with 200.0 g of
NH 3, as shown below? a) | 469.7 g | b) | 300.6 g | c) | 3.406
g | d) | 2.180 g |
|
|
|
10.
|
Hydrogen gas reacts with oxygen gas to form water as shown below: 2 H 2 (g) + O 2 (g)  2 H 2O
(l) 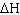 = -483.5 kJ What mass of hydrogen gas is required to produce 226 kJ
of heat? a) | 0.935 g | b) | 8.63 g | c) | 0.942
g | d) | 1.88 g |
|