Multiple Choice
|
|
|
1.
|
What mass of Mg is present in 25.5 g of magnesium chloride
(MgCl2)?
A) | 8.94 g | B) | 24.3 g | C) | 6.51
g | D) | 15.5 g |
|
|
|
2.
|
How many moles of CO 2 can be produced by the reaction of 5.00 mol of
C 2H 4 and 12.0 mol of O 2 as shown below? A) | 8.00 mol | B) | 10.0 mol | C) | 4.00
mol | D) | 5.00 mol |
|
|
|
3.
|
Which of the following is the correctly balanced equation for decomposition of
potassium chlorate (KClO3) to form potassium chloride and oxygen?
A) | KClO3 (s) 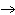 KCl (s) + 3 O
(g) KCl (s) + 3 O
(g) | B) | 2 KClO3 (s) 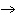 2 KCl (s) + 3 O2
(g) 2 KCl (s) + 3 O2
(g) | C) | 2 KClO3 (s) 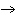 K2Cl2 (s)
+ 3 O2 (g) K2Cl2 (s)
+ 3 O2 (g) | D) | KClO3 (s) 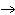 KCl (s) + O3 (g) KCl (s) + O3 (g) |
|
|
|
4.
|
How many grams of oxygen are needed to completely react with 200.0 g of
NH 3, as shown below? A) | 300.6 g | B) | 2.180 g | C) | 469.7
g | D) | 3.406 g |
|
|
|
5.
|
How many moles of water are produced when 2.50 moles of oxygen react with excess
hydrogen as shown below: A) | 1.25 mol | B) | 3.50 mol | C) | 2.00
mol | D) | 5.00 mol |
|
|
|
6.
|
The thermite reaction, shown below, is highly exothermic and used by railroad
repair crews to wels rails together. What mass of iron is formed when 725 kJ of heat are
released? A) | 65 g | B) | 130 g | C) | 95
g | D) | 47 g |
|
|
|
7.
|
What is the correct classification for the reaction shown below? A) | synthesis | B) | decomposition | C) | single
replacement | D) | double replacement |
|
|
|
8.
|
Cold packs, used in athletic and medical applications, work by lowering
temperature when ammonium nitrate is dissolved in water. Which of the following statements is
true for this process?
|
|
|
9.
|
How many moles of NH 3 are needed to react completely with 13.6 mol of
F 2, as shown below? A) | 5.44 mol | B) | 2.27 mol | C) | 6.80
mol | D) | 34.0 mol |
|
|
|
10.
|
Reduction can be defined as which of the following?
A) | gain of electrons | B) | loss of hydrogen | C) | gain of
oxygen | D) | all of these correctly define reduction |
|