Multiple Choice
|
|
|
1.
|
What type of reaction is shown below? CH 4 + 2 O 2 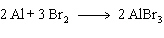
CO 2 + 2 H 2O + 218 kcal A) | Single replacement | B) | Endothermic | C) | decomposition | D) | Exothermic |
|
|
|
2.
|
The reaction 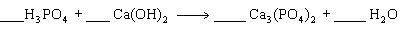 is an example of A) | single replacement | B) | double replacement | C) | combination | D) | decomposition |
|
|
|
3.
|
Calculate the number of moles in 112 g of aspirin,
C9H8O4.
A) | 0.619 | B) | 1.18 | C) | 0.165 | D) | 1.61 |
|
|
|
4.
|
Calculate the mass of 3.0 x 1022 atoms of sulfur.
A) | 160 g | B) | 0.16 g | C) | 1.6
g | D) | 16 g |
|
|
|
5.
|
In the reaction shown below, how many kcal of heat is required to produce 32.0 g
of O 2? A) | 274 kcal | B) | 548 kcal | C) | 68.5
kcal | D) | 137 kcal |
|
|
|
6.
|
In an endothermic reaction,
A) | energy is absorbed by the system. | B) | temperature of the surroundings
increases. | C) | heat flow out of the system. | D) | the products have less energy than the
reactants. |
|
|
|
7.
|
In the diagrams below, the green spheres represent chlorine atoms, yellow-green
spheres represent fluorine and the white spheres represent hydrogen atoms. Based on this
information, classify the reaction indicated below: A) | double replacement | C) | Single replacement | B) | Decomposition | D) | Synthesis |
|
|
|
8.
|
Which of the following describes an oxidation?
A) | loss of electrons or loss of oxygen | B) | loss of electrons or gain of
oxygen | C) | gain of electrons or gain of oxygen | D) | loss of electrons or gain of
hydrogen |
|
|
|
9.
|
Calculate the molar mass of
(NH4)3AsO4
A) | 193.03 g/mol | B) | 417.80 g/mol | C) | 156.96
g/mol | D) | 165.02 g/mol |
|
|
|
10.
|
When the equation shown below is balanced, the proper sequence of coefficients
for each substance is A) | 2, 3, 1, 6 | B) | 2, 3, 6, 1 | C) | 3, 2, 1,
6 | D) | 2, 3, 3, 1 |
|
|
|
11.
|
How many moles of NH 3 are needed to react completely with 2.00 mol of
O 2 accoding to the following equation: 4 NH 3
+ 3 O 2  2 N 2 + 6 H 2O A) | 6.00 mol | B) | 3.00 mol | C) | 2.67
mol | D) | 1.50 mol |
|
|
|
12.
|
If a mixture containing 3.0 mol N 2 and 8.7 mol H 2 are
allowed to react according to the reaction shown below: N 2 + 3 H 2  2 NH 3A) | H2 is limiting reactant | B) | only 2 mol of NH3 can be
produced | C) | N2 is limiting reactant | D) | 11.7 mol of NH3 can be
produced |
|
|
|
13.
|
How many atoms are present in 0.040 mol of O2?
A) | 2.4 x 1022 | B) | 4.8 x 1022 | C) | 1.5 x
1025 | D) | 6.6 x 1026 |
|
Short Answer
|
|
|
Balance each equation shown below:
|
|
|
14.
|
|
|
|
15.
|
|