Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
What is E 0 for the cell utilizing the following
half-reactions:: A) | -0.47 volts | B) | 0.47 volts | C) | 0.21
volts | D) | -0.21 volts |
|
|
|
2.
|
Which of the following processes results in a decrease in entropy?
A) | NH3 (l), –35 °C 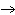 NH3 (g), –35
°C NH3 (g), –35
°C | B) | O2 (g) , 300 K 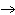 O2 (g), 400
K O2 (g), 400
K | C) | N2 (g), 25 °C 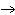 N2 (aq), 25
°C N2 (aq), 25
°C | D) | H2O (s), 0 °C 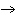 H2O (l), 0
°C H2O (l), 0
°C |
|
|
|
3.
|
In the voltaic cell shown below, which reaction occurs at the
cathode? Cu | Cu2+ || Ag+ |
Ag
|
|
|
4.
|
Which of the following conditions is sponatneous only at high
temperatures?
|
|
|
5.
|
Calculate 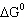 for the reaction:
| | | 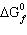 (kJ/mol) |
–24.5 | 0 | –394.4 |
–228.6 | | | | | |
A) | 598.5 kJ | B) | –598.5 kJ | C) | –2073.1
kJ | D) | 2073.1 kJ |
|
|
|
6.
|
Which of the following statements is true for the electrochemical
cells?
A) | The free energy change (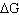 ) is negative for the voltaic cell. ) is negative for the voltaic cell. | B) | Oxidation occurs at
the cathode in the voltaic cell. | C) | The current flows from cathode to anode in both
cells. | D) | The free energy change (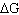 ) is negative for the electrolytic
cell. ) is negative for the electrolytic
cell. |
|
|
|
7.
|
Which letter represents the cathode in the cell notation shown
below: P | Q || R | S
|
|
|
8.
|
The voltaic cell made up of copper and metal M has E 0 = 0.62 V.
If E 0Cu = 0.34 V, what is E 0M? Cu 2+ (aq) + M (s)  Cu (s) +
M 2+ (aq) A) | –0.28 V | B) | –0.96 V | C) | 0.96
V | D) | 0.28 V |
|
|
|
9.
|
Predict the sign of 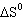 for the following
reaction: Pb (s) + Cl 2 (g)  PbCl 2 (s) A) | 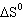 = 0 = 0 | B) | 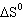 <
0 <
0 | C) | 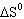 > 0 > 0 | D) | More information is
needed to make a reasonable prediction |
|
|
|
10.
|
Calculate 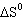 for the reaction shown
below:
| | | S° (J/Kmol) | 330.73 | 32.68 | 89.62 | 18.83 | | | | | |
A) | –198.02 J/K | B) | –330.73 J/K | C) | –254.96
J/K | D) | 254.96 J/K J/K |
|
|
|
11.
|
Calculate E 0cell and indicate whether the overall reaction
shown is spontaneous or non-spontaneous. 2 Cr (s) + 3
I 2 (s)  2 Cr 3+ (aq) + 6 I –
(aq) A) | E0cell = –1.28 V, non-spontaneous | B) | E0cell = –1.28 V, spontaneous | C) | E0cell = 1.28 V, non-spontaneous | D) | E0cell = 1.28 V, spontaneous |
|
|
|
12.
|
Hydrogen sulfide decomposes as shown below: For this reaction 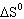 298 298 = 78.1 J/K, 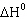 298 298 =
169.4 kJ, and 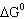 298 298 = 146.1 kJ. What is the value
of 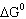 900 900? A) | 240 kJ | B) | 48.4 kJ | C) | 99.1
kJ | D) | –70100 kJ |
|
|
|
13.
|
Based on the reduction potentials given, which of the following substances is
the strongest reducing agent? A 2+ (aq) +
2 e –  A (s)
E 0 = –0.365 V B 2+ (aq) + 2 e –  B
(s) E 0 = –2.868 V C 2+
(aq) + 2 e –  C
(s) E 0 = 1.18 V D 2
(l) + 2 e –  2 D –
(aq) E 0 = 1.066 V
|
|
|
14.
|
The temperature at which the equilibrium below is established at 1 atm is the
normal melting point of phosphoric acid: Use the following data to determine this temperature. | Substance | H3PO4 (s) | H3PO4 (l) | 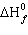 (kJ/mol) (kJ/mol) | –1284.4 | –1271.7 | 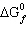 (kJ/mol) (kJ/mol) | –1124.3 | –1123.6 | | S° (J/K mol) | 110.5 | 150.8 | | | |
A) | 347 K | B) | 286 K | C) | 305
K | D) | 315 K |
|
|
|
15.
|
Which of the following species can act both as an oxidizing and a reducing
agent?
|