Multiple Choice
|
|
|
1.
|
Which of the following 0.1-M solutions is most acidic?
A) | pH = 7.22 | B) | pH = 8.34 | C) | pH =
11.88 | D) | None of the above; these solutions are all basic |
|
|
|
2.
|
What is the [H+] for a solution that has pOH =
5.640?
A) | 4.27 x 10–11 M | B) | 2.34 x 10–4
M | C) | 4.37 x 10–9 M | D) | 2.29 x 10–6
M |
|
|
|
3.
|
Benzoic acid (C6H5CO2H) is 1.0% ionized in a
0.010 M solution. What is the value of Ka for this acid?
A) | 1.0x10–3 | B) | 1.0x10–6 | C) | 1.0x10–8 | D) | 1.0x10–4 |
|
|
|
4.
|
Which of the following pairs has the stronger acid listed first?
A) | H2AsO3 and H2AsO4 | B) | HI and
HBr | C) | HClO and HClO2 | D) | H2S and
HCl |
|
|
|
5.
|
The following equilibrium is established with the
concentration of the products greater than the reactants:
H2C2O4 (aq) +
H2PO4– 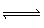 HC2O4– (aq) + H3PO4
(aq)
HC2O4– (aq) + H3PO4
(aq)
Which of the
following statements is true?
A) | The HC2O4– anion is a stronger base than
H2PO4– | B) | H2C2O4 is a
weaker acid than H3PO4 | C) | H3PO4 ia a weaker acid
than H2C2O4 | D) | H2PO4–
anion is a stronger acid than H2C2O4 |
|
|
|
6.
|
What is the pH of a 0.0085 M KOH solution?
A) | 11.93 | B) | 4.77 | C) | 9.23 | D) | 2.07 |
|
|
|
7.
|
Which of the following is NOT a conjugate acid-base pair?
A) | H3O+ / H2O | B) | HNO2 /
NO2– | C) | H2S /
S2– | D) | HSO4– /
SO42– |
|
|
|
8.
|
As the pH of a solution increases, its acidity ______________; as the pOH of a
solution increases, its acidity_______________
A) | increases; increases | B) | increases; decreases | C) | decreases;
increases | D) | decreases; decreases |
|
|
|
9.
|
A 0.20 M solution of a weak monoprotic acid has a pH of 3.00. The
ionization constant of this acid is
A) | 5.0x10–3 | B) | 2.0x10–7 | C) | 5.0x10–7 | D) | 5.0x10–6 |
|
|
|
10.
|
Each of the following can act as both a Bronsted-Lowry acid and base
except
A) | HCO3– | B) | NH4+ | C) | HS– | D) | H2PO4– |
|
|
|
11.
|
Which of the following correctly lists a conjugate acid-base pair,
respectively, in the reaction shown below: HSO 4– +
HCO 3– 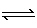 SO 42– +
H 2CO 3A) | HCO3– and
SO42– | B) | HSO4– and
HCO3– | C) | HSO4– and
SO42– | D) | HCO3– and
H2CO3 |
|
|
|
12.
|
What is the pH of a 0.050 M trimethylamine,
(C2H5)3N, solution? (Kb = 5.3x10-4)
A) | 8.68 | B) | 11.70 | C) | 10.50 | D) | 2.30 |
|
|
|
13.
|
The equilibrium constant for the reaction shown below is greater than 1.0.
Which of the following statements about the strength of the acids and bases is correct?
H 2PO 4–
+ HBO 32– 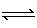 HPO 4–
+ H 2BO 3–A) | H2PO4– is weaker acid than
H2BO3– and HPO4– is
weaker base than HBO32– | B) | H2PO4–
is stronger acid than H2BO3– and
HPO4– is stronger base than
HBO32– | C) | H2PO4–
is stronger acid than H2BO3– and
HPO4– is weaker base than
HBO32– | D) | H2PO4–
is weaker acid than H2BO3– and
HPO4– is stronger base than
HBO32– |
|
|
|
14.
|
The term “Ka for the ammonium ion,
NH4+”, refers to which equation?
A) | NH4+ (aq) + OH– (aq) 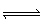 NH3 (aq) + H2O
(l) NH3 (aq) + H2O
(l) | B) | NH3 (aq) + H2O (l) 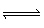 NH4+ (aq) + OH– (aq) NH4+ (aq) + OH– (aq) | C) | NH3 (aq)
+ H3O+ (aq) 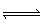 NH4+ (aq) + H2O (l) NH4+ (aq) + H2O (l) | D) | NH4+ (aq) + H2O (l) 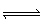 NH3 (aq) + H3O+
(aq) NH3 (aq) + H3O+
(aq) |
|
|
|
15.
|
Which of the following species in in the greatest concentration in a 0.10-M
solution of H2SO4?
A) | HSO4– | B) | SO42– | C) | H3O+ | D) | All species are in
equilibrium and therefore have the same concentrations |
|
|
|
16.
|
What is the pH of a 0.0100 M sodium benzoate solution? Kb
(C7H5O2–) = 1.5x10–10
A) | 5.91 | B) | 8.09 | C) | 0.38 | D) | 13.62 |
|