Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
1.
|
Decomposition of NO 2 occurs at 25 °C with K c= 1.83 x
10 –14. What is K p for this reaction? 2 NO 2 (g) 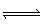
N 2 (g) + 2 O 2 (g) A) | 3.76 x 10–14 | B) | 8.92 x
10–15 | C) | 7.48 x
10–15 | D) | 4.48 x
10–13 |
|
|
|
2.
|
A chemical system at equilibrium will
A) | form more products if temperature is increased. | B) | have the same
concentration of all products and reactants. | C) | have a specific ratio of products to reactant
concentrations. | D) | not have any precipitates. |
|
|
|
3.
|
For the equlibrium shown below, which change will NOT be effective in increasing
the amount of N 2O 4 (g)? 2 NO 2 (g)
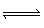 N 2O 4
(g) 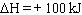 A) | adding more NO2 to the reaction vessel | B) | decreasing the
volume of reaction vessel | C) | removing N2O4 from
reaction vessel | D) | decreasing the temperature |
|
|
|
4.
|
The equilibrium constant (K c) for the decomposition of
COBr 2COBr 2 (g) 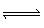 CO (g) + Br 2 (g) is
0.190. What is K c for the following reaction? 2 CO
(g) + 2 Br 2 (g) 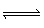 2 COBr 2
(g) A) | 10.5 | B) | 2.63 | C) | 27.7 | D) | 0.0361 |
|
|
|
5.
|
Cyclopropane is converted to propene in a first-order process. The rate
constant is 5.4 x 10–2 hr-1. If the initial concentration of
cyclopropane is 0.150 M, what will its concentration be after 22.0 hours?
A) | 0.105 M | B) | 0.0457 M | C) | 0.492
M | D) | 0.127 M |
|
|
|
6.
|
Given the two equlibria shown
below:
Cd 2+ (aq) + S 2O 32– (aq) 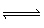 CdS 2O 3
(aq) K c= 8.3
x 10 3
CdS 2O 3 (aq) + S 2O 32– (aq) 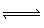 Cd(S 2O 3) 22–
(aq) K c= 2.5 x
10 2What the value for the equilibrium constant for the following reaction? Cd 2+ (aq) + 2 S 2O 32– (aq)
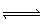 Cd(S 2O 3) 22– (aq) A) | 2.1 x 106 | B) | 8.1 x 103 | C) | 33 | D) | 8.6 x
103 |
|
|
|
7.
|
A fast reaction should have
A) | a low activation energy | B) | an exothermic heat of
reaction | C) | a large equilibrium constant | D) | a catalyst
present |
|
|
|
8.
|
The rate law for a particular reaction is found to be Rate = k[A]2[B]
Which of the
following actions will NOT change the initial reaction rate? A) | doubling the concentration of both A and B | B) | halving the
concentration of A and doubling the concentration of B | C) | doubling the concentration of A and halving the
concentration of B | D) | halving the concentration of A and quadrupling
the concentration of B |
|
|
|
9.
|
For which of the following will Kp = Kc?
A) | 2 NO (g) + O2 (g) 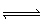 N2O4 (g)
N2O4 (g) | B) | MgCO3 (s) + 2 HCl (g) 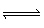 MgCl2 (s) + CO2 (g) +
H2O (l) MgCl2 (s) + CO2 (g) +
H2O (l) | C) | C (s) + O2 (g) 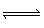 CO2 (g) CO2 (g) | D) | Zn (s) + 2 HCl
(aq) 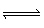 ZnCl2 (aq) + H2
(g) ZnCl2 (aq) + H2
(g) |
|
|
|
10.
|
A first order reaction has a half-life of 36 min. What is the value of
rate constant for this reaction?
A) | 1.9 x 10–2 s–1 | B) | 3.2 x
10–4 s–1 | C) | 1.2 s–1 | D) | 0.028
s–1 |
|
|
|
11.
|
In the reaction below, the rate of disappearance of H 2 is found to be
4.8 x 10 –2 M/s. What is the rate of disappearance of N 2 in this
reaction? N 2 (g) + 3 H 2
(g)  2 NH 3 (g) A) | 1.4 x 10–1 M/s | B) | 3.2 x 10–2
M/s | C) | 4.8 x 10–2 M/s | D) | 1.6 x 10–2
M/s |
|
|
|
12.
|
A mixture of 0.600 mol of bromine and 1.60 mol of
iodine is placed in a 1.0-L flask at 350°C. When the reaction
has come to equilibrium, the concentration of IBr is 1.19 M. What is the equilibrium constant
for this reaction?
Br2 (g) + I2 (g)  2 IBr
(g) 2 IBr
(g)
A) | 325 | B) | 3.55 x
10–3 | C) | 282 | D) | 1.47 |
|
|
|
13.
|
A reaction in which the rate and the rate constant have the same units is
a
A) | zero order reaction | B) | second order reaction | C) | first order
reaction | D) | radioactive decay |
|
|
|
14.
|
The kinetics of the decomposition of N2O5 is studied at
50°C and 75°C. Which of the
following statements concerning the study is correct?
A) | The rate at 75°C will be greater than the rate at
50°C because the activation energy will be lower at 75°C than at 50°C. | B) | The rate at 75°C will be greater than the rate at 50°C
because the number of molecules with enough energy to react increases with increase in
temperature. | C) | The rate at 75°C will be greater than the rate at
50°C because the concentration of the gas increases with increasing
temperature. | D) | The rate at 75°C will be lower than the rate at
50°C because the molecules at higher speeds do not interact as well
as those at lower speeds. |
|
|
|
15.
|
Which of the following CANNOT affect the extent of reaction?
A) | increasing the amount of reactants | B) | changing the temperature | C) | changing the
volume | D) | adding a catalyst |
|