Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
1.
|
Which of the following sets of factors affect the value of the rate constant,
k?
A) | reactant concentration and temperature | B) | reactant concentration and surface
area | C) | activation energy and reactant concentration | D) | temperature and
activation energy |
|
|
|
2.
|
The equilibrium constant, K p, for the reaction below is 55.2 at
425 °C. A rigid cylinder at this temperature contains 0.127 atm
of hydrogen, 0.134 atm of iodine and 1.055 atm of HI. Which of the following statements is
correct? H 2 (g) +
I 2 (g) 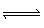 2 HI (g) A) | The forward reaction must proceed to establish equilibrium | B) | The system is at
equilibrium | C) | The reverse reaction must proceed to establish equilibrium | D) | more data is
required to draw a conclusion |
|
|
|
3.
|
At 500 °C the equilibrium constant,
K p, is 4.00 x10 –4 for the equilibrium: 2 HCN (g) 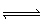 H 2
(g) + C 2N 2 (g) What is K p for the
following reaction? H 2 (g) +
C 2N 2 (g) 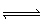 2 HCN (g) A) | 4.00 x 104 | B) | –4.00 x
10–4 | C) | 2.00 x
10–4 | D) | 2.50 x
103 |
|
|
|
4.
|
When the reaction A  B + C is studied,
a plot of log [A] vs. time gives a straight line with a negative slope. The order of
this reaction is A) | zero | B) | first | C) | third | D) | second |
|
|
|
5.
|
The decomposition of hydrogen peroxide is a first order reaction with a rate
constant of 1.06x10–3 min–1. How long will it take for the
concentration of H2O2 to drop from 0.0200M to 0.0120M?
A) | 4550 min | B) | 7.55 min | C) | 31400
min | D) | 482 min |
|
|
|
6.
|
For the reaction H2 (g) + I2
(g) 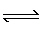 2 HI (g), at 700 K, Kc= 56.6. If an
equilibrium mixture at 700 K was found to contain 0.55 M HI and 0.21 M H2, then the
I2 concentration must be 2 HI (g), at 700 K, Kc= 56.6. If an
equilibrium mixture at 700 K was found to contain 0.55 M HI and 0.21 M H2, then the
I2 concentration must be
A) | 0.046 M | B) | 0.21 M | C) | 22
M | D) | 0.025 M |
|
|
|
7.
|
Nitrogen dioxide can dissociate into nitric oxide and oxygen:
2 NO 2
(g) 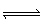 2 NO (g) + O 2
(g)  Under which reaction conditions would you expect to produce the
largest amount of oxygen? A) | high temperature, high pressure | B) | high temperature, low
pressure | C) | low temperature, high pressure | D) | low temperature, low
pressure |
|
|
|
8.
|
In which of the following equilibriums would the a change in volume have no
effect?
A) | N2 (g) + 3 H2 (g) 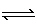 2 NH3
(g) 2 NH3
(g) | B) | NO (g) + O3 (g) 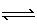 NO2 (g) +
O2 (g) NO2 (g) +
O2 (g) | C) | N2 (g) + 2 O2
(g) 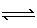 2 NO2 (g) 2 NO2 (g) | D) | N2O4 (g) 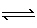 2 NO2
(g) 2 NO2
(g) |
|
|
|
9.
|
Which factor will not increase the concentration of ammonia as represented by
the equilibrium below? 3
H 2(g) + N 2 (g) 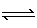 2 NH 3
(g) 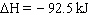
A) | increasing the concentration of N2 | B) | increasing the
pressure | C) | increasing the concentration of H2 | D) | increasing the
temperature |
|
|
|
10.
|
The rate law for the reaction 3A  2B is rate = k [A] with a
rate constant of 0.0447 hr –1. What is the half-life of the reaction if the
initial concentration of A is 0.500 M? A) | 6.25 hr | B) | 15.5 hr | C) | 0.0224
hr | D) | 44.7 hr |
|
|
|
11.
|
Considering the equilibrium shown below, which of the following changes will
increase the concentration of HgI 42–? HgO (s) + H 2O (l)  HgI 42– (aq) + OH –
(aq) A) | increasing the concentration of OH– | B) | increasing mass of
HgO | C) | adding a catalyst | D) | adding 6M
HNO3 |
|
|
|
12.
|
The decomposition of N 2O 5 has an activation energy of 102
kJ/mol and  H= +55 kJ/mol. What is the activation energy
for the reverse reaction? A) | 55 kJ/mol | B) | 157 kJ/mol | C) | 47
kJ/mol | D) | 102 kJ/mol |
|
|
|
13.
|
A catalyst is effective because
A) | it lowers the activation energy for the reaction. | B) | it increases the
number of collisions between the molecules. | C) | it increases the temperature of the molecules
in the reaction. | D) | it increases the number of molecules with enrgy equal to greater than the activation
energy. |
|
|
|
14.
|
For the reaction A + 2 B  2 C +
2 D, the following data were collected. Determine the rate law for this reaction.
Exp | [A], M | [B], M | Rate, M/min | 1 | 0.125 | 0.200 | 7.25 | 2 | 0.375 | 0.200 | 21.75 | 3 | 0.250 | 0.400 | 14.50 | 4 | 0.375 | 0.400 | 21.75 | | | | |
A) | Rate = k [A][B] | B) | Rate = k [A] | C) | Rate = k
[A][B]3 | D) | Rate = k
[A]2[B] |
|
|
|
15.
|
Reaction intermediates differ from activated complexes in that
A) | they are stable molecules with normal bonds and are frequently
isolated. | B) | they are unstable and can never be isolated. | C) | they are
intermediate structures which have characteristics of both reactants and
products. | D) | they are molecules with normal bonds rather than partial bonds and can occasionally
be isolated. |
|