Multiple Choice
Identify the choice that best completes the
statement or answers the question.
|
|
|
1.
|
16.55 mL of 0.844 M NaOH is required to titrate 10.0 mL of an unknown HCl
solution. What is the molarity of the HCl solution?
A) | 0.510 M | B) | 0.700 M | C) | 1.40
M | D) | 2.55 M |
|
|
|
2.
|
Which of the following ionic compounds is insoluble in
water?
A) | CuSO4 | B) | AgBr | C) | (NH4)2CO3 | D) | LiNO3 |
|
|
|
3.
|
What volume of H 2 at 675 mmHg and 25 ºC is required to produce
1.00 L of NH 3 gas at the same temperature and pressure as shown below: N 2 (g) + 3 H 2 (g)  2
NH 3 (g) A) | 3.00 L | B) | 1.00 L | C) | 1.50
L | D) | 2.00 L |
|
|
|
4.
|
The pressure of 4.0 L of nitrogen in a flexible container is decreased to
one-half of its original pressure, and its absolute temperature is increased to double its original
temperature. The volume is now
A) | 8.0 L | B) | 16 L | C) | 4.0
L | D) | 2.0 L |
|
|
|
5.
|
Determine the empirical formula for a compound that
contains C, H and O. It contains 52.14% C and 34.73% O by mass.
A) | C2H6O | B) | CH4O3 | C) | CHO | D) | CH3O |
|
|
|
6.
|
A gas sample at STP contains 1.15 g of oxygen and 1.55 g of nitrogen. What
is the volume of this gas sample?
A) | 2.04 L | B) | 4.08 L | C) | 1.26
L | D) | 61.0 L |
|
|
|
7.
|
Which of the following gases has the greatest density at 2.5 atm and 25 °C?
|
|
|
8.
|
The temperature of a gas in a sealed container changes from 40 °C to 80 °C. If the volume remains
constant, the pressure will change from 750 mmHg to
A) | 846 mmHg | B) | 375 mmHg | C) | 1500
mmHg | D) | 665 mmHg |
|
|
|
9.
|
What volume of 6.0 M NaOH is required to prepare 180 mL of 0.20 M solution of
NaOH?
A) | 6.0 mL | B) | 3.0 mL | C) | 18
mL | D) | 9.0 mL |
|
|
|
10.
|
Which of the following is a weak electrolyte in aqueous solutions?
|
|
|
11.
|
Which gas has the greatest root mean velocity at STP?
A) | Ar | B) | Ne | C) | He | D) | None of the above (all have the same kinetic
energy) |
|
|
|
12.
|
In which of the following sets do all species have
the same number of electrons?
A) | Br, Br-, Br+ | B) | K+,
Rb+, Cs+ | C) | Ge,
Se2-, Br- | D) | ) F-,
Ne, Mg2+ |
|
|
|
13.
|
A sample of He gas occupies 750 mL at STP. What is the volume will it
occupy at 780 mmHg and –77 ºC?
A) | 1.07 L | B) | 0.525 L | C) | 1.45
L | D) | 0.690 L |
|
|
|
14.
|
An ion that has 18 electrons and a charge of 2+
A) | has 16 electrons. | B) | has the same number of electrons as an Argon
atom. | C) | has 16 protons. | D) | is an anion. |
|
|
|
15.
|
How many protons, neutrons and electrons are present in the antimony (III) ion,
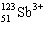 ? A) | 51, 72, 51 | B) | 123, 72, 123 | C) | 48, 72,
51 | D) | 51, 72, 48 |
|
|
|
16.
|
The balanced net ionic equation for reaction of calcium carbonate with nitric
acid is
|
|
|
17.
|
The correct name for the compound (NH4)2SO3
is
A) | ammonium sulfide | B) | ammonium sulfite | C) | ammonium
sulfate | D) | diammonium sulfite |
|
|
|
18.
|
How many grams of H3BO3 are needed to prepare 250 mL of
0.125 M H3BO3 solution?
A) | 1.93 g | B) | 0.505 g | C) | 3.09
g | D) | 0.312 g |
|
|
|
19.
|
What is the precipitate that forms when aqueous ammonium sulfide reacts with
aqueous copper (II) nitrate?
A) | CuS | B) | NH4NO3 | C) | NH4(NO3)2 | D) | Cu2S |
|
|
|
20.
|
What is the density (in g/L) of SO2 at 37 °C and 720 mmHg?
|